Close collaboration from idea to operation
At Kilde, we develop automation systems from scratch. We construct each system in close cooperation with the companies for which we are developing solutions. Our experts assist and advise throughout the process, ensuring that together we build a system that addresses your specific needs for efficiency.
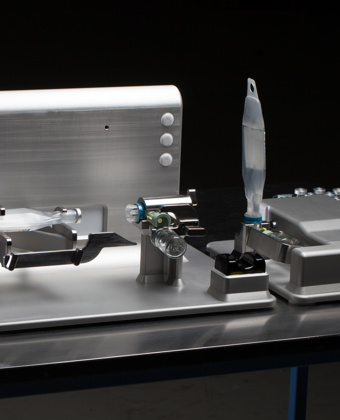
Streamlining Medico & Pharma
With tailored automation solutions for Medico & Pharma, processes become more reliable, faster, and less error-prone, which is essential in an industry where quality and safety are paramount.

Medico & Pharma
-
We develop complete solutions that optimize the manufacturing process from component assembly to advanced process solutions.
-
To ensure high production quality, quality control is carried out on the components and materials used in the final product – as well as on assemblies and processes both during and after production.
-
-
Our solutions collect and analyze production data in real time, providing you with valuable insights to optimize operations and ensure compliance with regulatory requirements.
-
We ensure that our automation solutions can seamlessly integrate with your existing systems and technologies, enabling rapid and efficient improvements.

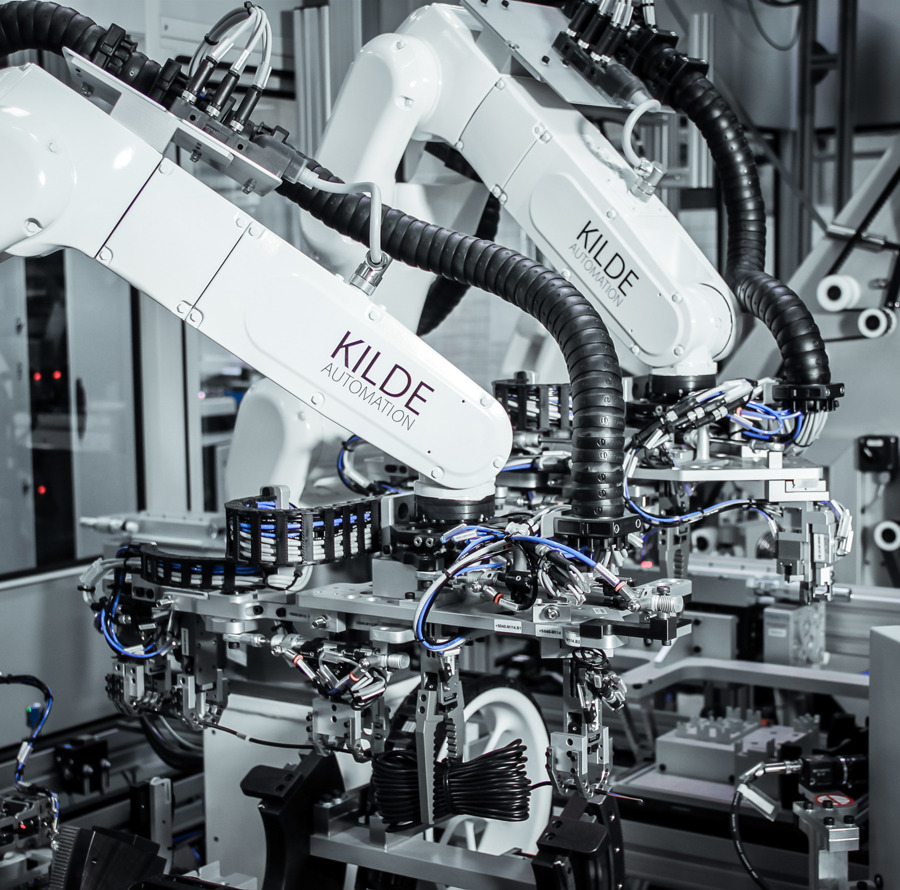
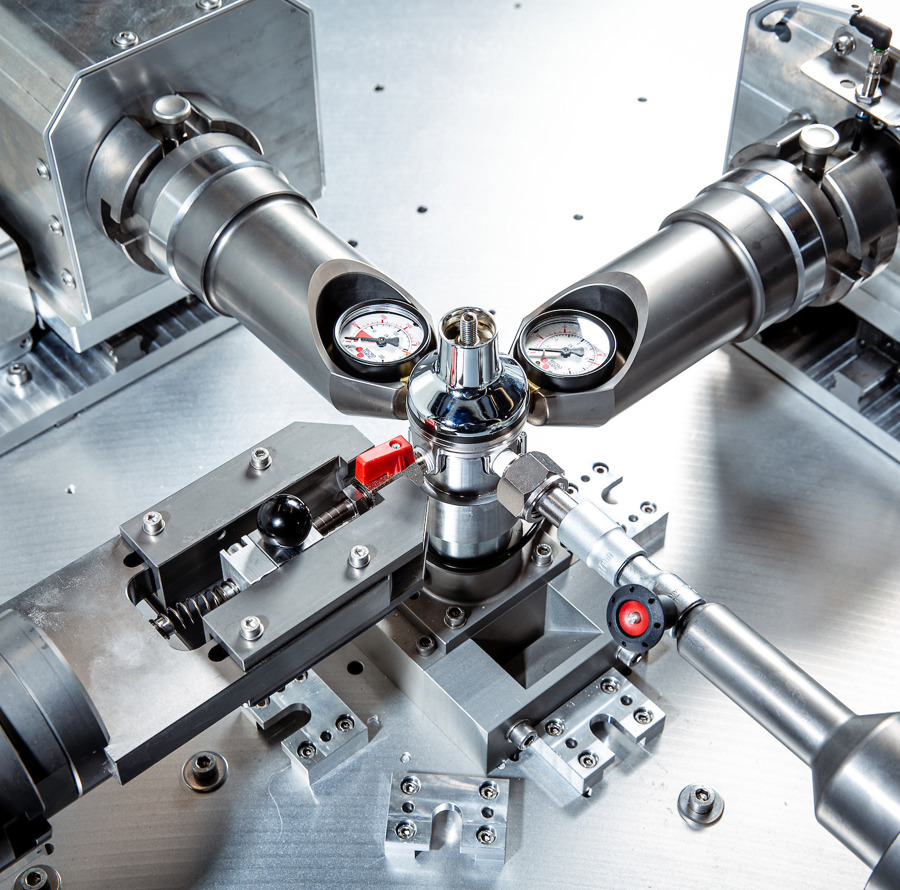


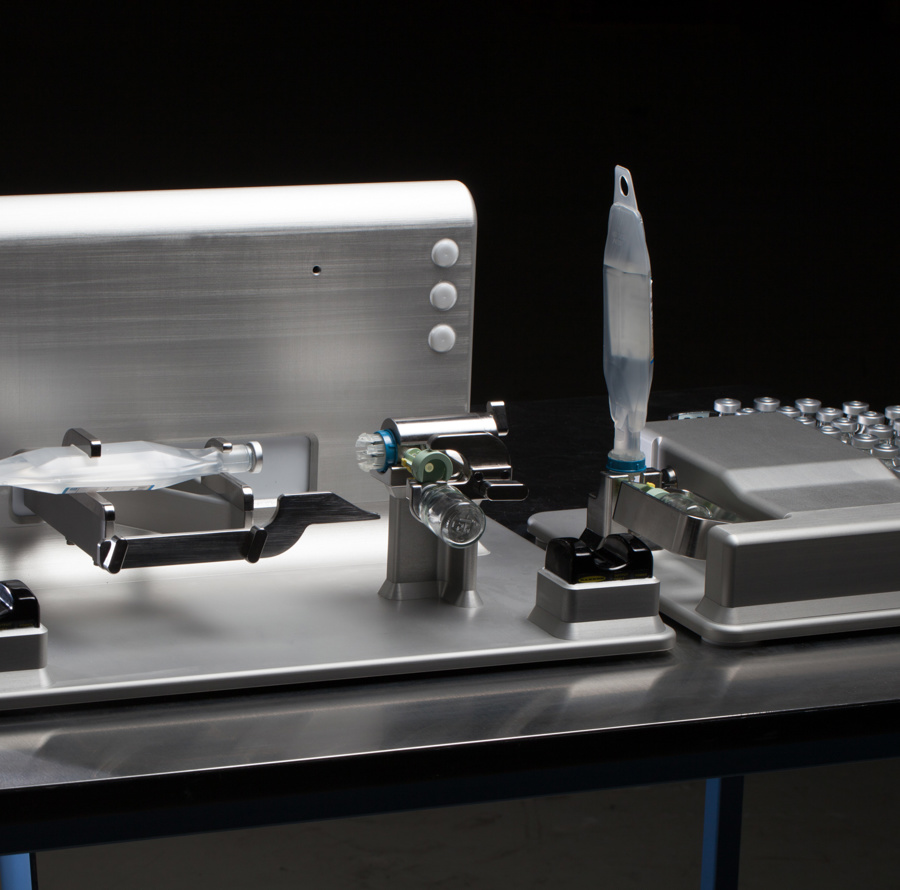
Documentation & Validation
At Kilde Automation, we take pride not only in delivering effective and innovative automation solutions but also in providing thorough documentation that supports our clients' needs for quality assurance and regulatory compliance. We understand that documentation is a central part of Medico & Pharma production, as it helps ensure traceability, compliance, and transparency throughout all project phases.
Requirement Specifications and Design
We document all relevant requirements and design descriptions so you get a clear overview of the project goals and the technical solution. This overview is anchored in the RTM (Requirement Traceability Matrix) and refers to documents such as FS (Functional Specification), HDS (Hardware Design Specification), SDS (Software Design Specification), and DQR (Design Qualification Report), which describe the technical details in depth.
Verification and Validation
We ensure that all systems and processes are thoroughly verified and validated according to test protocols that simplify quality assurance and quality control related to Installation Qualification (IQ) and Operational Qualification (OQ). These test protocols can be rigorously verified and validated in accordance with recognized methods and applicable standards such as GAMP 5, ISO 13485, and FDA regulations.
User and Maintenance Manuals
We prepare detailed user guides and maintenance documents that make it easy for your team to operate and maintain the automated solutions.
Traceability and Reporting
Our documentation supports full traceability of all processes and changes through version control, so you always have access to relevant information whenever needed.
GMP (Good Manufacturing Practice)
With many years of experience developing and implementing automated solutions that help our clients optimize production and ensure compliance with applicable regulations.
Kilde manages complex requirements and high standards within Quality Control (QC) and Quality Assurance (QA), which are crucial for ensuring reliable and dependable equipment operation.
Kilde works thoroughly to ensure compliance between equipment and the entire process surrounding validation and documentation.

Turnkey Systems
It is not necessary to have a finished concept or detailed specification to start a collaboration with us. We have extensive experience helping industrial companies identify their automation needs, and we are happy to assist you in designing and developing the project concept based on your requirements.
We deliver turnkey automation systems and take full responsibility throughout the entire project process — from specification to commissioning of the final solution.
We offer the following turnkey systems for the medico industry:
-
Assembly solutions
-
Process solutions
-
Packaging & palletizing

Automations solutions
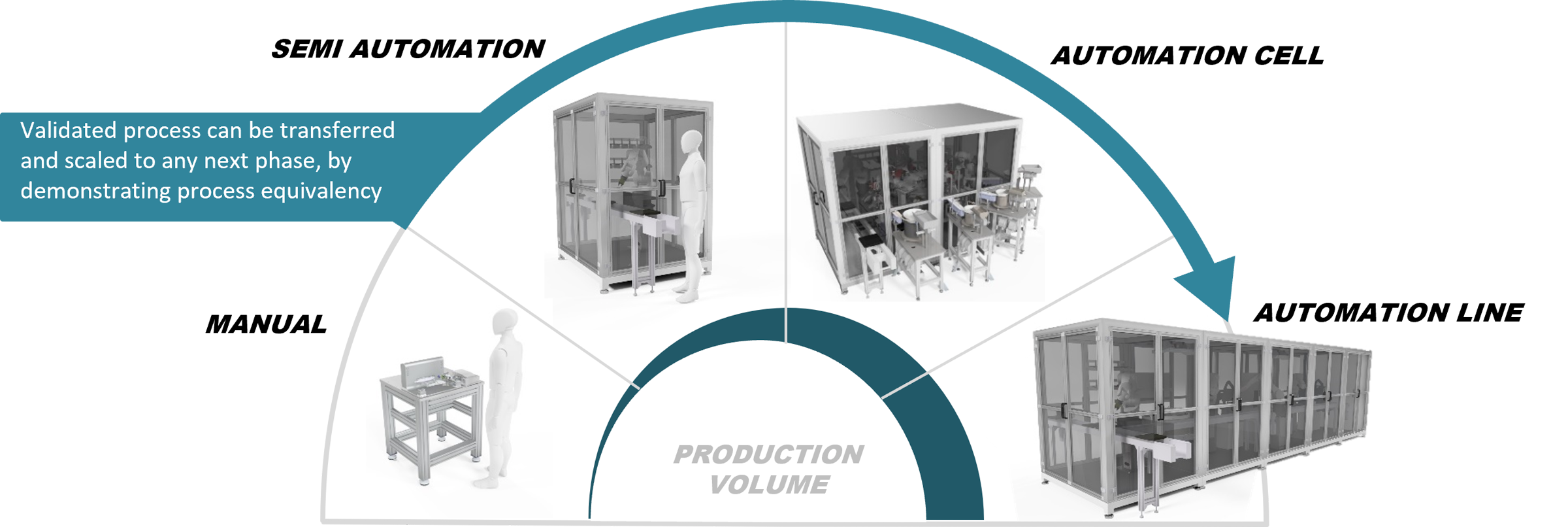
Our project model
Kilde is ISO 9001 certified. This means that you are ensured a solid foundation for quality management in the projects you undertake with us. We naturally have high demands for the process and constantly focus on optimizing and improving. We execute all projects with detailed descriptions of requirements for establishment, implementation, and maintenance.
We use the stage-gate model to manage and control the various phases of the project process. All phase-delimited deliveries in our projects are concluded with approval and evaluation before the next phase can commence. This ensures both transparency and optimal decision-making foundations, helping us stay on course for the project.
Our products for the medical industry
Watch videos of our automation projects nominated for DIRA-awards


Do you want to know more?
In close collaboration with your company, we assist with everything from needs analysis to implementation and commissioning of the final solution.
Do you have questions about how to get started with streamlining your production?

Rasmus Agerskov, Head of sales